Hey folks we need your Help!! Click Here
Scientific studies suggest zinc compound may be effective against coronavirus

Four studies from the University of Oxford, the American Society for Microbiology, the U.S. National Library of Medicine, and PLOS Pathogen outline the antiviral effects of zinc in the human body, including the immune system and respiratory system, and its efficacy against the common cold and coronavirus.
From Oxford Academic:
The Role of Zinc in Antiviral Immunity

ABSTRACT
Zinc is an essential trace element that is crucial for growth, development, and the maintenance of immune function. Its influence reaches all organs and cell types, representing an integral component of approximately 10% of the human proteome, and encompassing hundreds of key enzymes and transcription factors. Zinc deficiency is strikingly common, affecting up to a quarter of the population in developing countries, but also affecting distinct populations in the developed world as a result of lifestyle, age, and disease-mediated factors. Consequently, zinc status is a critical factor that can influence antiviral immunity, particularly as zinc-deficient populations are often most at risk of acquiring viral infections such as HIV or hepatitis C virus. This review summarizes current basic science and clinical evidence examining zinc as a direct antiviral, as well as a stimulant of antiviral immunity. An abundance of evidence has accumulated over the past 50 y to demonstrate the antiviral activity of zinc against a variety of viruses, and via numerous mechanisms. The therapeutic use of zinc for viral infections such as herpes simplex virus and the common cold has stemmed from these findings; however, there remains much to be learned regarding the antiviral mechanisms and clinical benefit of zinc supplementation as a preventative and therapeutic treatment for viral infections.
Introduction
Zinc deficiency was first recognized by Prasad et al. >50 y ago in a malnourished group of individuals presenting with hepatosplenomegaly, dwarfism, hypogonadism, and an elevated risk of infection (1). Unbeknownst to Dr. Prasad and his colleagues at the time, their discovery would highlight the importance of zinc as an integral component of human physiology, and inspire decades of zinc research. It is now understood that zinc is the second-most abundant trace metal in the human body after iron, and an essential component of protein structure and function. Importantly, zinc is a structural constituent of ∼750 zinc-finger transcription factors (2) enabling gene transcription, and is a catalytic component of approximately 2000 enzymes, encompassing all 6 classes (hydrolase, transferase, oxido-reductase, ligase, lyase, and isomerase) (3). Hence, zinc is biologically essential for cellular processes, including growth and development, as well as DNA synthesis and RNA transcription (4).
The global prevalence of zinc deficiency is estimated to range from ∼17% to 20% (5, 6), with the vast majority occurring in developing countries of Africa and Asia. Although significantly less common in high-income nations, zinc deficiency occurs most frequently in the elderly, vegans/vegetarians, and individuals with chronic disease such as liver cirrhosis (7) or inflammatory bowel disease (8). Importantly, zinc deficiency results in a compromised immune system, as evidenced by thymic atrophy, lymphopenia, and defective lymphocyte responses in animal studies (9). These data underscore the importance of zinc nutrition, particularly in underdeveloped countries where the risk of infection is heightened because of poor sanitation, public health, and vaccination strategies (5).
This review focuses on the role of zinc as an essential micronutrient that is required to mount an effective antiviral response. Although zinc possesses direct antiviral properties (e.g. influenza), it is also critical in generating both innate and acquired (humoral) antiviral responses. To complicate matters, zinc is an integral component of many viral enzymes, proteases, and polymerases, highlighting the importance of regulating cellular and systemic zinc distribution to inhibit viral replication and dissemination.
Current Status of Knowledge
Zinc homeostasis and viral infection
Systemic and intracellular zinc are tightly regulated, such that free zinc ions (Zn2+) represent a minimal fraction of total cellular zinc (∼0.0001%) (10–12). The vast majority of zinc remains bound to zinc-binding proteins such as serum albumin or intracellular metallothionein proteins, where it can be transferred to zinc-binding enzymes and transcription factors as necessary. Zinc transport is principally mediated by 2 groups of proteins: the ZnT [solute-linked carrier 30 (SLC30A)] family, which is responsible for efflux of zinc outside the cell or influx into organelles, and the ZIP [Zrt- and Irt-like proteins (SLC39A)] family of proteins, which performs the opposite role, transporting zinc into the cytoplasm from extracellular sources or cellular organelles (13). The >30 human proteins responsible for zinc homeostasis collectively ensure that zinc does not become toxic in the case of dietary excess, nor limited in the case of dietary insufficiency. Of course, this balance cannot be maintained indefinitely, and may result in zinc-induced copper deficiency if consumed in excess (14), and severe zinc deficiency if it is lacking in the diet (1).
Sequestration and toxic accumulation of metals are well-documented antibacterial immune responses. Calprotectin is a prime example, binding and sequestering extracellular calcium and zinc, thus preventing bacterial and fungal overgrowth (15). Conversely, toxic endosomal zinc accumulation can inhibit intracellular Mycobacterium growth in macrophages (16). Unfortunately, these mechanisms are not well described in the case of viral infections, perhaps because of a lack of efficacy. Calprotectin, for example, has no proven antiviral role, nor is it significantly upregulated in response to viral gastroenteritis (17). This absence of a zinc-mediated antiviral response may reflect the “parasitic” nature of viral infection, hijacking host machinery to self-replicate. Changes in intracellular zinc concentrations necessary to inhibit viral replication may also prove toxic to eukaryotic cells for the same reason.
Although antiviral modulation of zinc homeostasis in humans remains unproven, papilloma viruses have evolved mechanisms to alter zinc homeostasis to favor viral replication and persistence (18). The human papilloma virus (HPV) E5 protein can interact with the zinc transporter ZnT-1 in complex with EVER2, thus stimulating nuclear accumulation of zinc (19). The ZnT-1:EVER2 complex responsible for zinc export from the nucleus is inhibited by HPV E5, subsequently increasing both nuclear zinc and the activation of AP1 (20), a transcription factor required for HPV genome expression. Interestingly, homozygous mutations in either EVER1 or EVER2 result in a rare condition termed epidermodysplasia verruciformis (EV). EV patients are particularly susceptible to HPV strains 5 and 8, which significantly increases the risk of developing nonmelanoma skin cancers. HPV strains 5 and 8 lack expression of the E5 protein, which may explain 1) their limited replication in the normal population because of their inability to control zinc homeostasis, and 2) the susceptibility of EV patients to strains 5 and 8 from the loss of EVER protein function, favoring HPV replication. Interestingly, HPV E5 genes have co-evolved with the major HPV oncogenes, E6 and E7, and indicate the potential involvement of E5 in carcinogenesis (21, 22). Clinical trials using both oral and topical zinc have proven effective for the treatment of viral warts, and will be reviewed in a later section.
Metallothioneins, zinc homeostasis, and antiviral activity
Metallothioneins are small, cysteine-rich proteins capable of binding divalent cations such as zinc and copper. As vessels for much of the labile intracellular zinc pool, metallothioneins possess numerous functions through their ability to bind and release metals from their thiol groups. These include storage and transfer of zinc ions and heavy metal detoxification, as well as involvement in oxidative stress, apoptosis, and immune responses (23). Humans express 4 metallothionein isoforms (MT1–4), including the ubiquitously expressed MT1 and MT2 genes (MT1A, B, E, F, G, H, I, J, L, M, X, MT2A), as well as MT3 and MT4 whose expression is limited, and function remains poorly understood (24). Importantly, MT1 and 2 gene expression is extremely responsive to zinc, and therefore serves as an ideal indicator of an individual’s zinc status (25). Upon taking a zinc supplement, for example, an increase in protein-bound zinc in the bloodstream is internalized by cells in various tissues and organs through the ZIP transporters. In response to increased intracellular zinc, the metal-responsive transcription factor (MTF1) becomes active, and binds the metal responsive element in metallothionein gene promoters to upregulate their transcription (26). Although there are additional stimuli that influence metallothionein expression, this primarily occurs in a zinc-dependent fashion. Oxidative stress, for example, induces zinc release from metallothioneins as a mechanism to reduce reactive oxygen species generated by mitochondrial dysfunction or viral infection (26). Zinc released from metallothioneins binds MTF1 to stimulate additional metallothionein expression.
It should be noted that metallothioneins, although highly responsive to zinc, have long been classified as interferon stimulated genes (ISGs) (27). IFNs are immunostimulatory cytokines secreted from infected cells and nearby immune cells that induce the expression of hundreds of antiviral genes. They possess diverse roles including chemoattraction, immune cell activation, and direct antiviral activity. In response to IFNs, we suggest that there are 2 mechanisms of metallothionein induction. Most ISGs possess binding sites for STAT- or IFN regulatory factor (IRF) transcription factor-mediated expression, as is the case for MT1X and MT2A (28, 29). Other metallothioneins such as MT1F and MT1G do not possess known IFN regulatory regions in their promoters, but are instead more sensitive to zinc (28). IFNs stimulate an influx of zinc into the target cell, as is the case with some inflammatory cytokines such as IL-6, which in turn drives metallothionein expression.
Because metallothioneins possess such a diverse functional repertoire, their specific roles during viral infection remain undefined. However, both in vitro and in vivo studies have made it abundantly clear that metallothioneins are induced by viruses. The mechanisms often remain undefined; however, metallothionein expression has been attributed to zinc influx or redistribution (19, 28), by viral means, cytokine exposure, or oxidative stress (30). Metallothionein upregulation has been observed in response to measles virus (31), influenza (31, 32), HIV (33), hepatitis C virus (HCV) (34), and coxsackie virus (35), among others. In the case of HIV, zinc appears to be the key driver of metallothionein expression to favor viral persistence. HIV-infected monocytes demonstrate a significant increase in both MT1 gene expression as well as intracellular zinc (33). Elevated intracellular zinc increases monocyte resistance to apoptosis via inhibition of caspase 3 activation [as has been reported previously (36)], thus providing a reservoir for HIV replication. The role of metallothioneins remains unclear in this study; however, they have been described as negative regulators of apoptosis, albeit not through direct caspase 3 inhibition (37). Zinc and metallothioneins also facilitate human cytomegalovirus (HCMV) replication by activating the immediate-early HCMV promoter (38, 39). Kanekiyo et al. demonstrated that both zinc and metallothionein overexpression increased NF-κB binding in the HCMV promoter. Because no complex was detected between metallothionein and NF-κB, it was suggested that metallothioneins served as a zinc donor necessary for NF-κB binding. In addition, as NF-κB transcription factors are known potent activators of HIV and HSV replication, and several other viruses (40), metallothioneins may be proviral. Zinc has also been reported to inhibit NF-κB in numerous studies (41–43). Despite these contrasting data, Kim et al. have bridged these inconsistencies, demonstrating that MT2A can serve as a sink for excess zinc (44), thus limiting its proximity to NF-κB and favoring NF-κB-mediated transcription.
In the case of HCV infection, metallothioneins possess an antiviral role. Using a pan-metallothionein siRNA to knockdown all MT1 and 2 genes, we demonstrated both an increase in HCV replication and a decrease in intracellular zinc content in vitro (34). Interestingly, although ZnSO4 can reduce HCV replication, this effect was ablated when metallothionein genes were knocked down. These data suggest that metallothioneins are either 1) directly antiviral, potentially by sequestering zinc away from viral metalloproteins such as HCV NS5A (45), or 2) indirectly antiviral by acting as zinc chaperones and facilitating antiviral signaling. Further, metallothioneins possess antiviral properties against other viruses as well, as demonstrated in an antiviral screen of 380 human ISGs performed by Schoggins et al. (46). Overexpression of multiple members of the MT1 family inhibited replication of flaviviruses including yellow fever virus and HCV, as well as the alphavirus Venezuelan equine encephalitis virus. This effect was not observed in West Nile virus, and Chikungunya virus. These data indicate that metallothioneins, like many ISGs, are selectively antiviral, perhaps reflecting specific viral zinc requirements during replication. This is particularly evident for HIV, which demonstrated an increase in viral replication as a result of metallothionein overexpression in the Schoggins et al. ISG screen (46), validating previous works (33).
Zinc as an antiviral: bench to bedside and back again
Many studies have evaluated the efficacy of zinc as an antiviral agent in vitro. Unfortunately, zinc concentrations used to assess antiviral activity often far exceed physiological concentrations. Human plasma zinc, for example, ranges from approximately 10 to 18 µM (47), whereas antiviral concentrations of zinc can reach into mM concentrations (48). Intracellular zinc concentrations range from 10s to 100s of µM, but are significantly buffered by zinc-binding proteins such as metallothioneins, rendering free zinc concentrations at picomolar to low nanomolar concentrations (49, 50). The antiviral properties of zinc are certainly virus-specific, but it would appear that zinc ion availability plays a significant role in the antiviral efficacy of zinc (51). Here we describe the role of zinc as a virus-specific antiviral: both in vitro mechanistic studies, as well as human-based clinical trials using zinc supplementation. In vitro and in vivo studies are summarized in Tables 1 and 2, respectively.
Herpesviridae
The effect of zinc on HSV-1 and -2 has been studied for >40 y, with in vitro studies suggesting that zinc plays an inhibitory role on almost every aspect of the viral life cycle: viral polymerase function (52), protein production and processing (53), and free virus inactivation (48, 54). Although these studies were performed >20 y ago, a more recent study using the zinc ionophore pyrithione demonstrated a reduction in HSV replication from reduced NF-κB activation by interfering with the protein ubiquitination pathway (41). Unfortunately, no recent experimental data can demonstrate with any certainty the mechanism by which zinc inhibits HSV infection. Nonetheless, in vivo studies in mice and humans have shown a significant reduction of infection and disease burden. Mouse studies performing intravaginal zinc inoculation in liquid (55) or gel (56) form both resulted in significant reductions in HSV-2 infection. Several topical zinc application studies have been performed in humans, which demonstrated a significantly reduced recurrence and duration of infection (outbreak) (57–58). The efficacy of topical application, together with in vitro results (48, 54), suggest that free zinc may indeed coat HSV virions, thus preventing infection. Further research into this molecular mechanism is warranted.
Apart from HCMV mentioned above, the effect of zinc on other members of the Herpesviridae family remains unknown because of a lack of clinical data. Mechanistically, zinc ions have been shown to inhibit Varicella-Zoster virus by inactivating free virus in vitro (59). Both HSV and Varicella-Zoster virus belong to the Alphaherpesvirinae subfamily, reflecting their genetic relatedness, and similar mechanism of inhibition.


Picornaviridae
It was clear as early as 1974 that zinc possessed an inhibitory effect on picornovirus polyprotein processing (73). Before 1980, zinc inhibition of picornovirus proteases from human rhinovirus isolates (73, 74), encephalomyocarditis virus (62), poliovirus (61), and foot and mouth disease virus (64, 65) had all been demonstrated. More recent studies using zinc ionophores have illustrated that zinc interferes with the autocatalytic processing of the viral protease 3CDpro into 3Cpro in the picornavirus coxsackievirus B3, thus inhibiting processing of the viral polyprotein (107). However, this was not the case for encephalomyocarditis virus, where zinc appeared to inhibit the tertiary structure within the viral polyprotein (107). Together, these data suggest that zinc may interfere with proteolytic processing of the viral polyprotein because of misfolding, or through direct actions on the viral protease 3CDpro.
Clinical studies using zinc supplementation are primarily limited to rhinovirus infection, and are often grouped with other “common cold” viruses such as influenza and coronaviruses. The majority of studies use zinc lozenges with various zinc formulations and concentrations, possibly explaining the large variability in results [extensively reviewed in (108) and (109)]. Importantly, the amount of ionic zinc present at the site of infection (oral and nasal mucosa) is highly correlated to the study outcome (51, 108), and is dependent on the zinc formulation. At a physiological pH and 37°C, zinc gluconate for example, releases high amounts of ionic zinc, whereas zinc aspartate releases none (108). Upon examining only the relevant studies where high doses of ionic zinc were used, a clear reduction in cold duration of 42% was calculated (109). Whether this was caused by viral inhibition, improved local immune response, or an amelioration of symptoms remains uncertain.
Other respiratory tract infections: influenza, coronavirus, and metapneumovirus
Few studies have examined the antiviral effects of zinc on other respiratory viruses. In vitro replication of influenza (PR/8/34) is significantly inhibited by the addition of the zinc ionophore pyrrolidine dithiocarbamate (110), perhaps through inhibition of the RNA-dependent RNA polymerase (RdRp), as had been suggested 30 y earlier (111). In similar fashion, severe acute respiratory syndrome (SARS) coronavirus RdRp template binding and elongation was inhibited by zinc in Vero-E6 cells (60). Moreover, zinc salts were shown to inhibit respiratory syncytial virus, even while zinc was incubated with HEp-2 cells only before infection, and then removed (72). The authors suggest that this indicates an inhibitory mechanism similar to HSV by preventing viral membrane fusion; however, no measures were taken to assess changes in intracellular zinc content, nor inhibition of other aspects of the viral life cycle.
Flaviviridae: a focus on HCV
Flaviviruses represent a number of insect-borne viruses including dengue and West Nile virus, as well as the hepatotrophic virus, HCV. The effect of zinc on insect-borne flaviviruses is scarce; however, in vitro studies by our group (34) and others (67) have demonstrated that zinc salts can reduce HCV replication (∼50% at 100 µM ZnSO4), perhaps by inhibiting the HCV RdRp, as shown in E. coli [half maximal inhibitory concentration (IC50) ∼60 µM] (66). Although this is a potential mechanism, it has not been examined in eukaryotic cells in which zinc homeostasis is significantly different.
If left untreated, HCV becomes a chronic hepatic infection in around two-thirds of individuals (112), resulting in a significant reduction in plasma zinc (113). Consequently, zinc supplementation in HCV studies have focused on improved patient outcomes, particularly decreased liver inflammation, and enhanced response to antiviral treatment. Supplementation with 150 mg/d polaprezinc (a bioavailable zinc L-carnosine chelate) has been shown to reduce markers of hepatic inflammation alanine aminotransferase and aspartate aminotransferase alone (105), and in combination with the antiviral treatment IFN-α (106). Moreover, polaprezinc significantly improved the rate of viral clearance, particularly in patients with lower viral loads at baseline (102). The mechanisms underlying these observations remain uncertain; however, are likely a combination of direct antiviral effects and strengthening of the antiviral response. Zinc supplementation and the antiviral response is reviewed below.
Togaviridae
Like flaviviruses, togaviruses primarily consist of arthropod-borne viruses such as Semliki Forest virus, Western equine encephalitis virus, and Chikungunya virus. Viral infection occurs by receptor-mediated endocytosis, followed by fusion of virus and endosomal membranes, and particle release into the cytoplasm (114). Using liposome (76), red blood cell (115), and BHK-21 (77) cell model systems, zinc has been shown to efficiently inhibit membrane fusion of Semliki Forest virus and sindbis viruses. Zinc ions interfere with membrane fusion by binding to a specific histidine residue revealed on the viral E1 protein at low endosomal pH (77). Unfortunately, the in vivo relevance of this model is unclear because of the high concentration of zinc (>1 mM) used. Notably, concentrated zinc is present in vesicular zincosomes that are thought to serve as intracellular zinc storage vesicles (116). Similar to the mechanism used by macrophages to inhibit intracellular Mycobacterium spp., zincosome fusion to viral endosomes may inhibit key aspects of the viral life cycle such as togavirus membrane fusion.
Retroviridae: HIV
Retroviruses are named after their ability to transcribe RNA into DNA using their unique reverse transcriptase (RT), consequently allowing integration of retroviral DNA into the host genome. The integrated provirus can then establish a latent infection for the life of the host and is a major barrier to virus cure strategies, particularly for HIV-1 (117). Similar to viral RdRps, zinc has also been identified as an inhibitor of retrovirus RTs (118, 119). Fenstermacher and DeStefano demonstrated in 2011 that Zn2+ cations can displace Mg2+ ions from HIV-1 RT, promoting the formation of an excessively stable, but incredibly slow and inefficient replication complex (70). Zinc was also shown to inhibit the HIV-1 protease in 1991 (68), and to inhibit viral transcription in 1999 (69), but has received little attention since, with the exception of molecular simulation experiments that identified the zinc-binding sites at the catalytic aspartate-25 residue (120). As stated above, HIV can also stimulate zinc influx into monocytes (33), which may appear contradictory based on its antiretroviral properties. Latently infected monocytes and macrophages, however, can act as viral reservoirs for HIV (121), and could therefore benefit from zinc-mediated inhibition of cell death. In fact, unlike the majority of CD4+ T cells, low levels of replication in macrophages do not result in cell death (122), making them a viable reservoir, in addition to long-lived resting CD4+ T cells, for viral recrudescence after cessation of antiretroviral treatment.
Zinc deficiency is common in HIV-infected individuals, where it is associated with inflammation (123), immunological failure (124), and death (125). A recent Cochrane Review examined the role of micronutrient supplementation in people living with HIV (126). Although a number of studies demonstrated beneficial effects of zinc supplementation, the majority were underpowered. The authors concluded that zinc supplementation probably increases blood zinc concentration (moderate certainty), and may increase CD4+ counts (low certainty).
Unlike zinc supplements, prophylactic zinc gels have shown a substantial benefit to limit HIV infection in vivo. Complete protection against vaginal SHIV-RT (a simian HIV virus expressing the human RT) infection in macaques was obtained by pretreating animals with an antiviral gel containing 14 mM zinc acetate and 50 µM MIV-150, a reverse transcriptase inhibitor (127). When used alone, zinc acetate is a potent antiviral, providing 66% protection against SHIV-RT vaginal infection (56) and an EC50
Papillomaviridae
HPVs are oncogenic viruses that infect basal epithelial cells, where they stimulate proliferation resulting in warts. Although cutaneous warts are usually self-limiting and harmless, mucosal strains of HPV (e.g. high risk HPV-16 and -18) are a primary cause of cervical cancers (129). HPV oncoproteins E6 and E7 in particular, are significant drivers of cell proliferation and resistance to cell death by stimulating the degradation of tumor suppressor p53 and pRb, respectively [reviewed in (130)]. Although nuclear zinc appears to enhance HPV replication (see Zinc homeostasis and viral infection), exogenous zinc treatment (CIZAR, zinc chloride and citric acid anhydrous) can effectively inhibit production of viral oncogenic proteins E6 and E7 (71). The inhibition of E6 and E7 by zinc results in apoptosis of cervical carcinoma cells, as they regain the function of tumor suppressors p53 and pRb (71). The mechanism by which zinc downregulates E6 and E7 expression is unknown, but may be preceded by a zinc-driven blockade in another component of the viral life cycle.
It would appear that both topical and oral zinc supplementation strategies have proven tremendously effective for cutaneous and genital warts. Unfortunately, the vast majority of studies are either underpowered, lacking suitable controls, or single case studies. Nonetheless, a recent systematic review concluded that zinc supplementation was the most effective systemic treatment for cutaneous warts, when compared to other available options (131). It should be noted, however, that individuals with persistent viral warts are often zinc-deficient or have lower concentrations than their healthy counterparts (132). In fact, studies demonstrating the most significant responses to zinc treatment had engaged patients that were primarily zinc-deficient (>70 µg/dL) (92, 94). Nonetheless, 78% (94) and 100% (92) of patients showed clearance of lesions in response to oral zinc sulfate (10 mg/kg up to 600 mg/d) compared to 13% and 0% of the placebo group, respectively. Topical zinc formulations have also proved efficacious for treatment of viral warts. A small study using a 4-wk topical 10% zinc sulfate regimen for plane warts demonstrated an 86% response rate (6/7), compared to a 10% response rate (1/10) in the control group (93).
Recent work suggests that treatment of vaginal HPV infections with topical zinc formulations may benefit the millions of women that remain unvaccinated against HPV. A recent pilot study demonstrated that intravaginal infusion of 500 µM zinc citrate in women diagnosed with high-risk HPV resulted in a 64% clearance rate, compared to 15% in the control group (133). Additional studies in mice have demonstrated that MZC, a formulation containing MIV-50, zinc acetate, and carrageenan, efficiently inhibited vaginal and anorectal HPV-16 pseudoviral particle infection (134).
In summary, it is evident that zinc possesses antiviral properties against a number of viral species. Although mechanistic studies are lacking, zinc appears to inhibit viral protease and polymerase enzymatic processes, as well as physical processes such as virus attachment, infection, and uncoating (Figure 1). Unfortunately, these mechanisms have not been well scrutinized in clinical studies, where zinc may provide inexpensive and effective adjunct treatments for many viral infections.
The role of zinc in antiviral immune signaling
Ionic zinc possesses unique and distinct antiviral properties against a number of human viruses; however, the antiviral immune response led by IFNs is invariably required to clear infections. Zinc has been shown to contribute to a number of innate and adaptive immune signaling pathways that have been comprehensively reviewed recently (135). As such, this review will focus specifically on the role of zinc in the immune response to viruses.
Viral infections are recognized by a number of innate immune receptors termed pattern recognition receptors (PRRs). These include the cell surface and endosomal Toll-like receptors (TLRs), as well as a variety of cytosolic PRRs such as RIGI, MDA5, and IFI16 that primarily bind viral nucleic acids (136). Following ligand binding, PRRs share a number of downstream signaling intermediates, that ultimately activate both inflammatory (NF-κB, AP1) and innate immune (IRF1/3/7) transcription factors. These transcription factors cooperate to induce expression of IFNs, of which there are 3 types: type I (IFN-α and IFN-β), type II (IFN-γ), and type III (IFN-λs). Type I and III IFNs activate very similar antiviral signaling pathways; however, the type I IFN response is ubiquitous, whereas the type III IFN response is limited to a subset of immune cells, as well as epithelial cells of the liver, gastrointestinal, and pulmonary tracts (137). Although both IFN types bind unique receptors, they activate a common signaling cascade where STAT1 and STAT2 heterodimerize and bind IRF9, followed by translocation into the nucleus and subsequent binding of the IFN-sensitive response element that is present in hundreds of gene promoters. As stated previously, these ISGs possess numerous roles including immune cell chemotaxis and activation, as well as numerous antiviral mechanisms to inhibit viral replication within infected and neighboring cells.
Zinc and pathogen recognition
Upon recognition of microbial antigens by TLRs, a rapid and transient influx of free zinc ions occurs. Interestingly, this has been demonstrated in response to viral stimuli, imiquimod, ssRNA40 (TLR7), and CpG (TLR9), but not polyI: C (TLR3) in the mouse macrophage RAW 264.7 cell line (138). In response to TLR7 activation, zinc was shown to reduce the production of type I IFNs and ISGs CD80 and CD86. Based on results using other stimuli, the authors suggest that zinc can inhibit IRF3-, and perhaps IRF7-dependent IFNB production, by limiting activation and/or nuclear translocation (138). The role of the zinc influx in this context remains undefined, but may reflect a regulatory mechanism to prevent excessive IFN production.
Although no direct inhibition of IRF signaling by zinc has been demonstrated, zinc can modulate a number of factors upstream of IRF activation. For example, the IκB kinase (IKK) members IKKα and IKKβ are inhibited by zinc, albeit at high concentrations of ∼0.5 µM (139). IKKα has been shown to activate IRF7 in response to TLR7/9 stimulation (140), whereas IKKβ (141), IKKε and TANK-binding kinase-1 (TBK1) (142) can activate IRF3 following TLR3 stimuli. Zinc can also stimulate expression of the deubiquitinating enzyme A20 (43) to inhibit the pathogen response. A20 is a regulator of NF-κB- (143), TLR3- (144), and RIGI-mediated (145) IFN production, most likely by targeting PRR signaling components TIR-domain-containing adapter-inducing interferon-β (TRIF), TNF Receptor Associated Factor (TRAF) 2, and TRAF6. A20-deficient cells are hyper-responsive to viral infection, possess increased activation of NF-κB, IRF3, and IRF7, and improved viral clearance (146).
Zinc and the interferon response
After pathogen recognition, NF-κB, AP1, and IRF3/7 bind IFN promoters to stimulate type I/III IFN production. Zinc plays a significant role in the response to IFNs by modulating secretion, cytokine potency, and receptor binding, as well as influencing signaling intermediates and pathway inhibitors. A recent study has demonstrated that intracellular zinc can reduce IFN secretion by destabilizing sortilin mRNA transcripts (147). Sortilin is an endosomal protein that facilitates secretion of cytokines such as IFN-γ and IL6 (148), and its depletion results in a significant reduction in secretion of IFN-α. Consequently, because sortilin ensures trafficking and secretion of numerous cytokines, it is possible that zinc also inhibits the secretion of other IFNs.
Structural studies have demonstrated that zinc ions can mediate dimerization of IFNA molecules (149). Nonetheless, apart from crystallization studies, dimers were difficult to generate despite using concentrated IFN (50 µM) and zinc (1 mM). It is therefore likely that the circulating active form of IFN-α is monomeric. A single study performed in 2001 showed that zinc can increase the antiviral activity of IFN-α 10-fold against rhinovirus challenge (150). Although this study drew radical conclusions, antiviral activity was based on cytopathic effect alone, and its results have not been reproduced since. Moreover, zinc was added before viral infection, which is known to interfere with rhinovirus polyprotein processing (73, 74), as reviewed above.
Unlike type I IFNs, a recent study by our group has shown that zinc can inhibit IFN-λ3 signaling, most likely by preventing receptor binding and subsequent signaling (28). Upon demonstrating in 2014 that metallothionein expression was IFNL genotype-dependent, and inversely associated with ISG expression in HCV (151), we showed that serum zinc was the driver of hepatic metallothionein expression. Although zinc had minimal effect on IFN-α signaling, it could almost ablate IFN-λ3 signaling at a concentration of 50 µM, resulting in a significant reduction in its antiviral activity (28). Interestingly, we found no inhibition of IFN-λ1 activity using 50 µM ZnSO4, suggesting a highly specific interaction. The mechanism by which zinc interferes with the IFN:receptor interaction remains uncertain; however, we have ruled out an effect of zinc on IFN-λ3 disulfide bond formation.
Type I and III IFNs bind to unique receptor complexes composed of IFN-α receptors IFNAR1/IFNAR2 and IFN-λ receptors IFNLR1/IL10RB, respectively, but signal via almost identical pathways. Consequently, zinc may act to reinforce the shared IFN signaling cascade by inhibiting protein tyrosine phosphatase enzymatic activity (152). Following receptor engagement by IFNs, intracellular Janus protein tyrosine kinases Jak1 and Tyk2 become phosphorylated, which in turn phosphorylate STAT molecules to stimulate ISG expression. By dephosphorylating these key signaling molecules, a number of phosphatases have been shown to “put the brakes” on IFN signaling. Phosphatases tyrosine-protein phosphatase non-receptor type 6 (SHP1), type 11 (SHP2), and protein phosphatase 2A (PP2A) have all been shown to inhibit JAK-STAT phosphorylation (153–155), and are all inhibited by zinc ions, predominantly in the nanomolar range (156–158). Interestingly, PP2A can also inhibit the phosphorylation of IRF3, thus regulating antigen recognition by PRRs (159). Conversely, the tumor suppressor phosphatase and tensin homologue (PTEN) stimulates IRF3 activation by removing inhibitory phosphorylation at Ser97 (160), and is also inhibited by zinc at nanomolar concentrations (161). Zinc inhibits numerous pro- and antiviral phosphatases, with the net effect on virus recognition and response being undefined, which clearly requires further study.
To enable a highly controlled IFN response, negative regulators of IFN signaling are often ISGs. These include the suppressors of cytokine signaling (SOCS-1 and SOCS-3), which bind and inhibit JAK protein signaling, thus preventing signaling from numerous inflammatory (IL-6) and antiviral stimuli (162). Interestingly, zinc-driven activation of the MTF-1 transcription factor can induce expression of SOCS-3 in HepG2 cells (163). The zinc importer ZIP-14, which is responsible for zinc influx following inflammatory stimuli, was required for SOCS-3 expression, and may represent yet another zinc-mediated mechanism to limit the inflammatory response. Although the transporter responsible for hepatic zinc influx following IFN stimulation remains unknown, it is perceivable that ZIP-14 may drive zinc influx and subsequent SOCS-3 expression.
Zinc deficiency caused by disease, age, and lifestyle factors: lessons from supplementation
Zinc status is primarily determined by dietary zinc intake; however, additional factors such as dietary composition, alcohol intake, and disease state can significantly reduce zinc uptake and storage, or increase zinc excretion (164). With respect to dietary composition, zinc supplementation as part of a meal can significantly reduce zinc absorption when compared to water-based solutions of zinc (164). Moreover, dietary phytate, a natural chelator of zinc ions that is present in corn, rice, and cereals, can severely restrict zinc absorption (165). Consequently, diets containing high phytate: zinc molar ratios, can result in zinc deficiency, even with adequate zinc intake. Unfortunately, rural diets in low-income nations are often zinc-poor and phytate-rich because of a dietary reliance on rice and vegetables.
Aged individuals are also significantly more susceptible to zinc deficiency, increasing their likelihood of acquiring life-threatening viral infections (166). Ex vivo, zinc supplementation has been shown to improve leukocyte IFN-α production (167) and to reduce mononuclear cell TNF production (168). Year-long supplementation with 45 mg elemental zinc/d in elderly subjects (aged 55–87 y), has also demonstrated a dramatic reduction in the incidence of infection as well as plasma oxidative stress markers (168).
Alcoholism can stimulate severe zinc deficiency developed via numerous sociological and physiological mechanisms, with factors including but not limited to 1) increased urinary zinc excretion (169), 2) reduced zinc intake (poor diet) (170), 3) reduced zinc absorption (171), and 4) and a reduction in hepatic zinc stores (172). Alcohol also stimulates microbial dysbiosis and gastrointestinal permeability (173), a phenotype that can increase the likelihood of viral infection in the gut (174). Importantly, dietary zinc supplementation can improve intestinal barrier dysfunction as a result of alcohol and microbial infection (175, 176).
As previously discussed, zinc deficiency is common among chronic infections such as HPV, HCV, and HIV (113, 123). Consequently, a number of studies have examined the effects of zinc supplementation on antiviral immunity, inflammation, and treatment response. As described above, zinc supplementation can improve HCV treatment response and liver inflammation caused by chronic infection. In addition, long-term zinc treatment over 7 y has been shown to reduce the risk of hepatocellular carcinoma progression in chronic HCV patients, as assessed by multivariate analysis, compared to controls (P
Vaccination studies
Zinc supplementation during vaccination strategies has provided an opportunity to examine the role of zinc in the humoral response to viruses. A particular focus has been applied to the effect of zinc supplementation on rotavirus vaccination because of the high rate of mortality associated with childhood diarrhea in developing countries. Unfortunately, although zinc deficiency is associated with increased risk of rotavirus gastroenteritis (178), it does not greatly increase the development of humoral immunity followed by vaccination (rotarix), as defined by seroconversion rate (179). Nonetheless, a pooled analysis of randomized trials performed in 2000, demonstrated that zinc supplementation shortens the length of diarrheal episodes and reduced the rate of treatment failure or death by 42% in zinc-deficient children (180).
Comparable studies of supplementation with zinc before vaccination have produced similar disappointing results. Zinc supplementation did not improve seroconversion following administration of the oral poliovirus vaccine in infants (181), nor did it improve the immunological response to HBV (182) or influenza vaccination (183) in the elderly. Although there remains little evidence that zinc improves viral vaccination responses, a small number of studies suggest that zinc may improve antibody titers and antibacterial responses to pneumococcus (184) and cholera infections (185).
Conclusions and Future Perspectives
The tight regulation of zinc homeostasis both systemically and intracellularly indicates that zinc plays an essential role in human health. Although zinc is a component of ∼10% of the human proteome, zinc in different forms (free compared with protein-bound) can stimulate a variety of signaling events, including the antiviral response. In vitro studies suggest that free zinc may possess potent antiviral effects, and are supported by trials of creams, lozenges, and supplements with high free zinc content. Moreover, zinc-binding proteins such as the metallothioneins may possess antiviral roles, although their specific function remains uncertain. Nonetheless, zinc treatment applied at a therapeutic dose and in the right form has the potential to drastically improve the clearance of both chronic and acute viral infections, as well as their accompanying pathologies and symptoms. Consequently, the role of zinc as an antiviral can be separated into 2 categories: 1) zinc supplementation implemented to improve the antiviral response and systemic immunity in patients with zinc deficiency, and 2) zinc treatment performed to specifically inhibit viral replication or infection-related symptoms (75, 78–82, 83, 85–91, 95–101, 103, 104).
From American Society for Microbiology:
Effect of Zinc Salts on Respiratory Syncytial Virus Replication

ABSTRACT
Zinc supplementation decreases the morbidity of lower respiratory tract infection in pediatric patients in the developing world. We sought to determine if zinc mediates a specific inhibitory effect against the major cause of pediatric lower respiratory tract disease, respiratory syncytial virus (RSV). We determined the in vitro inhibitory effect of three zinc salts (zinc acetate, lactate, and sulfate) on the replication of RSV at various concentrations of 10 and 1 mM and 100 and 10 μM. The degree of inhibition of RSV replication was examined in the presence of zinc during preincubation, adsorption, or penetration and was compared with that caused by salts of other divalent cations. Complete inhibition of RSV plaque formation was observed at 1 and 10 mM, representing reductions that were ≥106-fold. At the lowest concentration tested, 10 μM, we observed ≥1,000-fold reductions in RSV yield when zinc was present during preincubation, adsorption, penetration, or egress of virus. The therapeutic indices, determined as ratios of 50% toxicity concentration to 50% inhibitory concentration, were 100, 150, and 120 for zinc acetate, zinc lactate, and zinc sulfate, respectively. The inhibitory effect of zinc salts on RSV was concentration dependent and was not observed with other salts containing divalent cations such as calcium, magnesium, and manganese. RSV plaque formation was prevented by pretreatment of HEp-2 cell monolayer cultures with zinc or by addition of zinc to methylcellulose overlay media after infection. The results of this study suggest that zinc mediates antiviral activity on RSV by altering the ability of the cell to support RSV replication.
Respiratory syncytial virus (RSV) is the most important viral cause of acute respiratory tract infection (ARI) in infancy and early childhood, with its greatest morbidity in infants (6, 8, 9). Throughout the world, RSV contributes significantly to annual hospital admissions due to ARI, especially during the epidemic season (18, 23, 27). In developing countries, malnutrition and deficiencies of micronutrients are associated with increased incidence and severity of ARI (25). Improvement in nutritional status might be a practical strategy for prevention of severe RSV disease in developing countries, if efficacious. At this time, however, we have a poor understanding of the role of micronutrients such as zinc in susceptibility to severe disease caused by RSV infection.
Zinc supplementation in children in developing countries was demonstrated to cause a significant reduction in the prevalence of pneumonia (3, 20). Zinc has been shown to mediate antiviral effects against certain viruses. Clinical studies showed that zinc significantly shortens the duration of symptoms during rhinovirus infection (17, 19, 28). Topical application of zinc sulfate also was found in one study to be effective in the treatment of herpes simplex virus (HSV) infection (26). The specific mechanism by which zinc mediated these clinical effects is unknown in most cases. Zinc also enhances the host response to many infections and plays an important role in the homeostasis of the immune system (12, 21, 24). Zinc deficiency, often seen in the context of malnutrition, is associated with an impaired cellular immune response (2, 4).
Several studies have examined the direct inhibitory effect of zinc on viruses that infect the human respiratory tract, including the enveloped virus HSV and the nonenveloped virus rhinovirus (7, 13, 14). The mechanism of inhibition of rhinovirus likely involves direct binding of zinc to virus particles (13). Zinc also inhibits the replication of human immunodeficiency virus type 1 (HIV-1) (10), vaccinia virus (11), and polioviruses (15) in vitro. Although zinc supplementation reduces the incidence of severe clinical pneumonia in the developing world, the effect of zinc on replication of the primary agents of viral pneumonia in infants such as RSV is unknown. We determined the in vitro inhibitory effect of three zinc salts (acetate, lactate, and sulfate) on RSV in virus yield or virus plaque reduction assays by treatment of wild-type RSV strain A2 at various concentrations of 10 and 1 mM and 100 and 10 μM. Because of the dramatic effect of reduction of RSV when zinc was present throughout the virus replication cycle, we also sought to determine the stage of virus replication that was inhibited. The degree of virus inhibition was examined in the presence of zinc during preincubation with the cell culture monolayer or during viral adsorption, penetration, or budding and was compared with that mediated by salts of other cations at similar concentrations. The experiments suggest that zinc inhibits RSV by altering the ability of cells to support RSV replication rather than by a direct effect on the virus.
MATERIALS AND METHODS
Cells and virus HEp-2 cell monolayer cultures were maintained in Opti-MEM I medium (Life Technologies, Gaithersburg, Md.) supplemented with glutamine, amphotericin B, gentamicin, and 5% fetal calf serum. Opti-MEM I medium, which contains less than 3.4 nM zinc in the form of zinc sulfate, was used as mock treatment for the experiments. Working stocks of the virus were prepared by propagation in HEp-2 cell monolayer cultures at 37°C after infection at a multiplicity of infection (MOI) of 0.1 PFU/cell. When a significant cytopathic effect was detected, the supernatant was collected, clarified, aliquoted, and stored in cryovials at −70°C until used. The endpoint plaque titer of the virus stock used was 5.3 log10 PFU/ml.
Zinc solutions USP-grade zinc acetate (AMEND Chemical, Irvington, N.J.), or zinc lactate or sulfate (Sigma Chemical, St. Louis, Mo.), was used in all experiments. Calcium acetate, magnesium sulfate, and manganese sulfate (Sigma Chemical) were used as divalent cation control salts. Stock solutions of 250 mM salts were prepared in deionized water and were sterilized by passage through a 0.22-μm-pore-size syringe tip filter.
Zinc cytotoxicity The cytotoxic effect of zinc salts on HEp-2 cell monolayer cultures was assessed by addition of salts in cell culture media to 90% confluent cell culture monolayers in a 24-well plate and by incubation at 37°C for 96 h. At the end of the incubation period, cells were trypsinized and were gently resuspended in medium and cell viability was assessed with trypan blue exclusion.
Treatment with zinc salts An equal volume of virus was combined with various concentrations of zinc salt or control salts in 50 mM morpholinepropanesulfonic acid (MOPS)-buffered Opti-MEM I medium, pH 7.2, in a total volume of 250 μl, and the mixture was incubated at 37°C for 2 h. The MOPS buffer was used to prevent alteration or significant variation of pH due to various zinc concentrations, which otherwise would be a confounding variable. Control wells without zinc were treated similarly with MOPS-buffered Opti-MEM I medium. At the end of the incubation period, the zinc-virus mixture was diluted in medium with or without zinc according to the experimental design, and the plaque assay was performed as described below.
Plaque reduction assay Serial 10-fold dilutions of zinc-virus mixtures were performed. One hundred microliters of suspension was transferred to 90% confluent HEp-2 cell monolayer cultures in 24-well plates (Costar, Cambridge, Mass.). Virus was allowed to adsorb for 2 h at 37°C, overlaid with a semisolid medium containing 0.75% methylcellulose (Sigma Chemical), and incubated at 37°C. At 96 h postincubation, the cells were fixed in 80% methanol at 4°C for 1 h. RSV plaques were stained by an immunoperoxidase staining method with murine RSV F glycoprotein-specific monoclonal antibodies (clones 1269, 1243, and 1129), goat anti-mouse immunoglobulin G-horseradish peroxidase conjugate, and an appropriate precipitating substrate (4CN) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). In plaque assays, the virus was diluted to give approximately 90 PFU per well in the absence of inhibitory salts. In addition, some assays were performed during which HEp-2 cell monolayer cultures were pretreated with zinc or control salt for 2 h at 37°C prior to infection with RSV. Experiments were also performed in which zinc salts were present in the experiment only in the semisolid overlay media that was added to the culture after virus absorption and penetration.
Effect of zinc on viral yield HEp-2 cell monolayer cultures were infected with RSV at an MOI of 0.1 PFU/cell and were allowed to adsorb for 2 h at 37°C. Nonadsorbed virus was removed by washing with phosphate-buffered saline (PBS) three times, and the medium was replaced with medium containing the desired concentration of zinc or control salt. After 96 h, virus was harvested by collecting supernatants from the cultures and was frozen in cryovials and stored at −70°C pending determination of viral titer.
Effect of zinc on viral penetration To determine the effect of zinc on viral penetration, HEp-2 cell monolayer cultures were prewashed with ice-cold PBS, infected with RSV at an MOI of 0.1 PFU/cell, and then incubated at 4°C for 2 h. Nonabsorbed virus was removed by washing three times with cold PBS. Medium containing a desired concentration of zinc or control salt was added to the monolayer cultures and was further incubated at 37°C for 4 h. Excess zinc or control salt was removed by washing with PBS and was replaced with complete Opti-MEM I medium without added salts. After a 96-h period of incubation, supernatants containing virus were harvested from the cultures and were frozen in cryovials at −70°C pending determination of viral titer.
Statistics Data analysis was performed with SPSS for Windows version 10.0 statistical software (Chicago, Ill.). Percent cell viability was determined by comparing the number and proportion of cells viable in zinc-treated cell culture monolayers to those in mock-treated monolayers. Percent of RSV plaque counts in the presence of zinc or control salts were determined by normalizing the plaque counts for the mock-treated wells to 100%. Mean values were compared by the analysis of variance and Student t test where appropriate. A statistically significant difference was defined as a P of
RESULTS
Zinc toxicityFigure 1 shows the cytotoxicity of zinc salts on HEp-2 cell monolayer cultures. The toxicity concentration (TC50) for HEp-2 cells was 3, 5, or 7.5 mM for zinc sulfate, acetate, or lactate, respectively. Zinc concentrations between 10 and 100 μM did not mediate a cytotoxic effect for HEp-2 cell monolayer cultures that could be detected by visual inspection with light microscopy. Cytotoxicity as determined by cell viability with the trypan blue dye exclusion test was observed at 1 and 10 mM, although more than 50% of the cell monolayer was intact at those concentrations.

Zinc inhibits RSV in plaque reduction assay. (i) Effect of zinc on RSV when present throughout adsorption, penetration, and egress(a) Virus yield. When zinc was present during adsorption, penetration, and egress, there was significant reduction in virus yield, even at the lowest concentration of 10 μM, when comparisons were made to mock-treated or control salt-treated wells (Fig. 2). The mean virus titer in the mock-treated wells was 5.3 log10 PFU/ml (standard error of the mean [SEM] = 0.7 log10 PFU/ml) compared to 2.8 log10 PFU/ml (SEM = 0.7 log10 PFU/ml) for any of the zinc salts (P

(b) Plaque formation. To further determine the effect of treating RSV with zinc salts, we examined the effect of zinc on RSV plaque formation under a semisolid overlay, which requires cell-to-cell spread in culture. Figure 3 shows the plaque counts after treatment of RSV with zinc or control salts in a plaque reduction assay with approximately 90 PFU of RSV per well. The inhibitory effect of zinc salts on RSV was concentration dependent and was significantly greater than that during treatment with calcium, magnesium, or manganese salts (P

(ii) Effect of zinc on viral penetrationThe inhibitory effect of zinc salts on RSV penetration of cells was evaluated (Fig. 4) by separating the process of adsorption from penetration by using temperature shifts, ensuring that zinc salts were added only during viral penetration. Figure 4 shows the effect of zinc and control salts on RSV penetration. A significant inhibitory effect by zinc salts on viral penetration was observed at 100 μM, 1 mM, or 10 mM when compared to the effect caused by control salts. A statistically significant effect was not observed with a 10 μM concentration of any of the zinc salts. The mean virus titer in the mock-treated cultures was 6.6 log 10 PFU/ml (SEM, 0.2 log10 PFU/ml).

(iii) Effect of zinc on viral yield after absorptionIn order to determine the effect of zinc salts on viral replication (Fig. 5), virus yields were determined in the presence of zinc salts added only after viral adsorption. A significant reduction in viral yield was observed at 1 mM in the presence of zinc salts, with a mean titer of 1.6 log10 PFU/ml (SEM, 0.2 log10 PFU/ml) for any zinc salt compared to 4.7 log 10 PFU/ml (SEM, 0.3 log10 PFU/ml) in mock-treated or control salt-treated cultures (P

(iv) Effect of zinc on plaque formation when applied only before or only after absorptionAddition of zinc salt to the semisolid overlay media after infection significantly prevented RSV plaque formation by preventing cell-to-cell spread (Fig. 6). When HEp-2 cell monolayer cultures were pretreated with zinc prior to infection of the monolayer with 90 PFU of virus/well, a concentration-dependent inhibitory effect was observed with zinc salt compared to control salts. The difference was significant even at the lowest zinc concentration tested, 10 μM (P = 0.03) (Fig. 7).

DISCUSSION
The results of our study demonstrate that zinc salts inhibit RSV in vitro. The inhibition is concentration dependent and specific. The salts of other divalent cations, such as calcium, magnesium, and manganese, do not mediate a similar inhibition. At the lowest concentration of 10 μM, we were able to observe a 800-fold reduction in virus when zinc was present during preincubation, adsorption, penetration, and egress. Similar to the behavior that we observed, zinc ions inhibited in vitro replication of viruses such as rhinovirus (13), HSV (1), HIV (10), and vaccinia virus (11). Both laboratory-adapted and clinical isolates strains of HSV were inhibited by 100 μM zinc sulfate (1, 14). The degree of inhibition of these viruses was influenced by the duration of treatment with zinc salt. However, complete inhibition was observed at a concentration 50 to 5,000 times higher than observed in our study. Zinc compounds at 100 μg/ml inhibited HIV infection in vitro (10), though an effect on HIV syncytium formation was not observed at this concentration. This finding is in contrast to our observation, i.e., that zinc not only inhibited RSV replication but also prevented plaque formation, even when added in the semisolid overlay media only after virus infection of the cell monolayer. We observed a difference in the magnitude of the inhibition of plaque formation and that of virus yield, with virus yield more significantly inhibited. Previous studies have demonstrated that the mechanisms of viral budding and viral attachment and fusion with cells differ from the mechanisms involved in cell-cell fusion. Also, the yield experiments were performed under conditions in which multicycle growth curves were studied, which may have accentuated the magnitude of virus inhibition. The inhibitory effect that we observed was not due simply to cytotoxic effect on the culture monolayer, since more than 50% of the area of the cell culture monolayer was intact during the plaque assay.
Zinc salts also prevented RSV plaque formation when present on the cell monolayer only prior to viral infection. The significant effect of zinc salts on viral yield and penetration observed in this study suggests that the inhibitory effect of zinc salt is mainly due to an alteration of the ability of cells to support viral replication. Previous electron microscopy studies with HSV demonstrated massive deposition of zinc onto HSV virions, which interfered with viral glycoprotein-mediated membrane fusion (15). In contrast, the inhibitory effect of zinc salts on RSV that we observed in this study is likely due to deposition of zinc on or in the cell monolayer, which prevented viral adsorption and subsequent plaque formation. This idea is supported by the observation of reduction in plaque formation when only the HEp-2 monolayer cells were pretreated with zinc prior to infection with RSV. It has been previously demonstrated that there is minimal zinc uptake into cultured cells treated in vitro (16). We also observed that addition of zinc to semisolid overlay media containing methylcellulose inhibited efficient RSV infection by preventing cell-to-cell spread in culture. This observation further suggests a principal effect on cells, rather than on virions in solution. These data do not completely rule out a contribution of direct binding of zinc to virion particles, since in some of the assays both cells and virion particles were exposed to zinc. Nevertheless, the data as a whole show that the dominant effect is that on the cell substrate.
The molecular mechanism of antiviral effect of zinc is not clear in these studies. It has been proposed that zinc may act in several ways to inhibit viruses (15). Zinc may block the binding of rhinovirus virions to the cell surface by fitting into the canyons on the surface of the virus, thereby preventing attachment to the cell. Alternatively, zinc may block the protease activity in rhinovirus, thereby preventing the breakdown of the virus polypeptide necessary to generate individual functional proteins. Zinc compounds owe their anti-HIV-1 effects to inhibition of HIV-1 DNA-to-RNA transcription, rather than inhibition of the adsorption or penetration, contrary to our observation on RSV in this study (10).
Our study indicates that HEp-2 cell monolayer cultures, a cell line derived from a human laryngeal carcinoma, appear to be more tolerant of high concentrations of zinc than are many other cell lines that have been examined in vitro. The TC50 for zinc salts was between 3 and 7.5 mM in our study. This finding is in contrast to the previous observation that zinc concentrations more than 200 μM were toxic to the monkey cell line CV-1, human neonatal kidney cells, or the human diploid lung fibroblast cell line MRC-5 (16). Zinc salts at a concentration of 1 mM or 300 μM caused extensive cellular toxicity with destruction of uninfected MRC-5 fibroblast monolayer cell cultures after 1 day of exposure (16). The TC50 of zinc compounds on human T-cell lines was between 8 and 550 μM; severe toxicity was observed at concentration of 1 mM (16). The tolerance of the HEp-2 cell line for zinc enabled us to study the inhibitory effect of zinc salts on RSV at a broader range of concentrations than did previous studies of zinc inhibition of rhinovirus, HSV, or HIV.
Zinc mediates a therapeutic effect in vivo in certain viral infections. While some clinical studies showed that zinc significantly shortened the duration of symptoms during rhinovirus infection (5, 17), other studies showed that zinc had no therapeutic effect (22, 28). Topical application of zinc sulfate has also been found effective in the treatment of HSV infection in one study (17). In vitro studies have also demonstrated inactivation of both HSV types 1 and 2 by zinc salts (1, 14). The therapeutic relevance of zinc in RSV infection is not clear. Our studies were not designed to examine therapy per se. The physiological level of zinc in plasma is 10 to 20 μM (24), and only about 10 to 20% of the ingested dose of zinc is absorbed. Although we observed reduction in infectivity with 10 μM zinc, complete inhibition was only seen at a concentration about 100-fold higher than the normal plasma zinc level. To achieve systemic concentrations at such levels with oral zinc therapy likely would be difficult without resulting local zinc toxicity such as mucosal ulceration. However, during supplementation and correction of deficiency in zinc-deficient states, the proportion of supplemental zinc that is absorbed increases (4). This mechanism may explain partially the decrease in incidence of ARI and pneumonia observed in children under 5 years of age with moderate-dosage zinc supplementation in certain developing countries (3). The reduction in incidence of ARI in those populations might also be due to other factors, such as an improvement in immune function.
Zinc enhances host resistance to infection and plays a critical role in homeostasis of the immune system. Zinc deficiency is associated with an impaired cellular immune response and may play a role in the reduced cellular immunity seen in malnutrition (4, 19, 21). An imbalance between Th1 and Th2 functions has been reported during zinc deficiency (2, 19). In human studies, zinc deficiency results in decreased production of interleukin 2 (IL-2) and gamma interferon (cytokines associated with Th1 cells), whereas the production of IL-4, -6, and -10 (cytokines associated with Th2 cells) is not affected (2).
In conclusion, zinc salts inhibited RSV in vitro when present during preincubation, adsorption, penetration or egress. In addition, zinc salts prevented efficient RSV infection by preventing cell-to-cell spread in culture when the HEp-2 cell monolayer cultures were pretreated with zinc only prior to infection with RSV or when zinc was added to semisolid overlay media only after infection. The inhibitory effect was concentration dependent and was not observed during treatment with salts of other divalent cations. The inhibitory effect appears to be due to an effect of zinc salts on epithelial cell ability to support virus replication rather than a direct effect on the virus itself. Studies that examine the role of zinc deficiency and infant zinc supplementation on RSV infection may be important, especially among populations with zinc deficiency. In the absence of zinc deficiency, however, zinc treatment of RSV infection likely would not be tolerated at the concentrations that were observed by us to inhibit RSV replication in this study.
From the U.S. National Library of Medicine:
Efficacy of Zinc Against Common Cold Viruses: An Overview

ABSTRACT
Objective: To review the laboratory and clinical evidence of the medicinal value of zinc for the treatment of the common cold.
Data sources: Published articles identified through Medline (1980-2003) using the search terms zinc, rhinovirus, and other pertinent subject headings. Additional sources were identified from the bibliographies of the retrieved articles.
Study selection: By the author.
Data extraction: By the author.
Data synthesis: Human rhinoviruses, by attaching to the nasal epithelium via the intracellular adhesion molecule-1 (ICAM-1) receptor, cause most colds. Ionic zinc, based on its electrical charge, also has an affinity for ICAM-1 receptor sites and may exert an antiviral effect by attaching to the ICAM-1 receptors in the rhinovirus structure and nasal epithelial cells. Clinical tests of zinc for treatment of common colds have been inconsistent, primarily because of study design, blinding, and lozenge contents. Early formulations of lozenges also were unpalatable. In three trials with similar study designs, methodologies, and efficacy assessments, zinc effectively and significantly shortened the duration of the common cold when it was administered within 24 hours of the onset of symptoms. Recent reports of trials with zinc gluconate administered as a nasal gel have supported these findings; in addition, they have shown that treatment with zinc nasal gel is effective in reducing the duration and severity of common cold symptoms in patients with established illness.
Conclusion: Clinical trial data support the value of zinc in reducing the duration and severity of symptoms of the common cold when administered within 24 hours of the onset of common cold symptoms. Additional clinical and laboratory evaluations are warranted to further define the role of ionic zinc for the prevention and treatment of the common cold and to elucidate the biochemical mechanisms through which zinc exerts its symptom-relieving effects.
Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture

From PLOS Pathogens:
ABSTRACT
Increasing the intracellular Zn2+ concentration with zinc-ionophores like pyrithione (PT) can efficiently impair the replication of a variety of RNA viruses, including poliovirus and influenza virus. For some viruses this effect has been attributed to interference with viral polyprotein processing. In this study we demonstrate that the combination of Zn2+ and PT at low concentrations (2 µM Zn2+ and 2 µM PT) inhibits the replication of SARS-coronavirus (SARS-CoV) and equine arteritis virus (EAV) in cell culture. The RNA synthesis of these two distantly related nidoviruses is catalyzed by an RNA-dependent RNA polymerase (RdRp), which is the core enzyme of their multiprotein replication and transcription complex (RTC). Using an activity assay for RTCs isolated from cells infected with SARS-CoV or EAV—thus eliminating the need for PT to transport Zn2+ across the plasma membrane—we show that Zn2+ efficiently inhibits the RNA-synthesizing activity of the RTCs of both viruses. Enzymatic studies using recombinant RdRps (SARS-CoV nsp12 and EAV nsp9) purified from E. coli subsequently revealed that Zn2+ directly inhibited the in vitro activity of both nidovirus polymerases. More specifically, Zn2+ was found to block the initiation step of EAV RNA synthesis, whereas in the case of the SARS-CoV RdRp elongation was inhibited and template binding reduced. By chelating Zn2+ with MgEDTA, the inhibitory effect of the divalent cation could be reversed, which provides a novel experimental tool for in vitro studies of the molecular details of nidovirus replication and transcription.
Author Summary
Positive-stranded RNA (+RNA) viruses include many important pathogens. They have evolved a variety of replication strategies, but are unified in the fact that an RNA-dependent RNA polymerase (RdRp) functions as the core enzyme of their RNA-synthesizing machinery. The RdRp is commonly embedded in a membrane-associated replication complex that is assembled from viral RNA, and viral and host proteins. Given their crucial function in the viral replicative cycle, RdRps are key targets for antiviral research. Increased intracellular Zn2+ concentrations are known to efficiently impair replication of a number of RNA viruses, e.g. by interfering with correct proteolytic processing of viral polyproteins. Here, we not only show that corona- and arterivirus replication can be inhibited by increased Zn2+ levels, but also use both isolated replication complexes and purified recombinant RdRps to demonstrate that this effect may be based on direct inhibition of nidovirus RdRps. The combination of protocols described here will be valuable for future studies into the function of nidoviral enzyme complexes.
Introduction
Zinc ions are involved in many different cellular processes and have proven crucial for the proper folding and activity of various cellular enzymes and transcription factors. Zn2+ is probably an important cofactor for numerous viral proteins as well. Nevertheless, the intracellular concentration of free Zn2+ is maintained at a relatively low level by metallothioneins, likely due to the fact that Zn2+ can serve as intracellular second messenger and may trigger apoptosis or a decrease in protein synthesis at elevated concentrations [1], [2], [3]. Interestingly, in cell culture studies, high Zn2+ concentrations and the addition of compounds that stimulate cellular import of Zn2+, such as hinokitol (HK), pyrrolidine dithiocarbamate (PDTC) and pyrithione (PT), were found to inhibit the replication of various RNA viruses, including influenza virus [4], respiratory syncytial virus [5] and several picornaviruses [6], [7], [8], [9], [10], [11]. Although these previous studies provided limited mechanistic information, this suggests that intracellular Zn2+ levels affect a common step in the replicative cycle of these viruses.
In cell culture, PT stimulates Zn2+ uptake within minutes and inhibits RNA virus replication through a mechanism that has only been studied in reasonable detail for picornaviruses [11], [12]. In vitro studies with purified rhinovirus and poliovirus 3C proteases revealed that protease activity was inhibited by Zn2+ [13], [14], which is in line with the inhibition of polyprotein processing by zinc ions that was observed in cells infected with human rhinovirus and coxsackievirus B3 [11]. The replication of segmented negative-strand RNA viruses such as influenza virus, however, does not depend on polyprotein processing and the effect of PDTC-mediated Zn2+ import was therefore hypothesized to result from inhibition of the viral RNA-dependent RNA polymerase (RdRp) and cellular cofactors [4]. Moreover, an inhibitory effect of Zn2+ on the activity of purified RdRps from rhinoviruses and hepatitis C virus was noted, but not investigated in any detail [15], [16].
Details on the effect of zinc ions are currently largely unknown for nidoviruses. This large group of positive-strand RNA (+RNA) viruses includes major pathogens of humans and livestock, such as severe acute respiratory syndrome coronavirus (SARS-CoV), other human coronaviruses, the arteriviruses equine arteritis virus (EAV), and porcine reproductive and respiratory syndrome virus (PRRSV) [17], [18]. The common ancestry of nidoviruses is reflected in their similar genome organization and expression strategy, and in the conservation of a number of key enzymatic functions in their large replicase polyproteins [19]. A hallmark of the corona- and arterivirus replicative cycle is the transcription of a 5′- and 3′-coterminal nested set of subgenomic (sg) mRNAs from which the viral structural and accessory protein genes are expressed [20], [21].
Analogous to picornaviruses [13], [22], zinc ions were demonstrated to inhibit certain proteolytic cleavages in the processing of the coronavirus replicase polyproteins in infected cells and cell-free systems [23], [24]. In this study we report that the zinc-ionophore pyrithione (PT) in combination with Zn2+ is a potent inhibitor of the replication of SARS-coronavirus (SARS-CoV) and equine arteritis virus (EAV) in cell culture. To assess whether – besides a possible effect on proteolytic processing – nidovirus RTC subunits and RNA synthesis are directly affected by Zn2+, we employed in vitro systems for SARS-CoV and EAV RNA synthesis that are based on membrane-associated RTCs isolated from infected cells (from here on referred to as RTC assays) [25], [26]. In addition, we used in vitro recombinant RdRp assays to directly study the effect of zinc ions on the RdRps of SARS-CoV and EAV [27], [28].
Using these independent in vitro approaches, we were able to demonstrate that Zn2+ directly impairs nidovirus RNA synthesis, since it had a strong inhibitory effect in both RTC and RdRp assays. Interestingly, the Zn2+-mediated inhibition could be reversed through the addition of a Zn2+ chelator (MgEDTA). We therefore applied this compound to stop and restart the in vitro RNA-synthesizing activity at will. This convenient tool allowed us to study various mechanistic aspects of arteri- and coronavirus RNA synthesis in more detail. Additionally, the zinc-mediated inhibition of nidovirus RNA synthesis described here may provide an interesting basis to further explore the use of zinc-ionophores in antiviral therapy.
RESULTS
Zinc and pyrithione inhibit nidovirus replication in vivo
Zinc ions are involved in many different cellular processes, but the concentration of free Zn2+ is maintained at a relatively low level by metallothioneins [1]. Zn2+ and compounds that stimulate import of Zn2+ into cells, such as PT, were previously found to inhibit replication of several picornaviruses, including rhinoviruses, foot-and-mouth disease virus, coxsackievirus, and mengovirus in cell culture [6], [7], [8], [9], [10], [11]. To determine whether Zn2+ has a similar effect on nidoviruses, we investigated the effect of PT and Zn2+ on the replication of EAV and SARS-CoV in Vero-E6 cells, using reporter viruses that express green fluorescent proteins (GFP), i.e., EAV-GFP [29] and SARS-CoV-GFP [30]. EAV-GFP encodes an N-terminal fusion of GFP to the viral nonstructural protein 2 (nsp2), one of the cleavage products of the replicase polyproteins, and thus provides a direct readout for translation of the replicase gene. In SARS-CoV-GFP, reporter expression occurs from sg mRNA 7, following the replacement of two accessory protein-coding genes (ORFs 7a and 7b) that are dispensable for replication in cell culture.
We first assessed the cytotoxicity of a range of PT concentrations (0–32 µM) in the presence of 0 to 8 µM ZnOAc2. Treatment with PT of concentrations up to 32 µM in combination with

Using these non-cytotoxic conditions we subsequently tested the effect of PT and ZnOAc2 on EAV-GFP and SARS-CoV-GFP replication. To this end, Vero-E6 cells in 96-well plates were infected with a multiplicity of infection (m.o.i.) of 4. One hour post infection (h p.i.), between 0 and 32 µM of PT and 0, 1, or 2 µM ZnOAc2 were added to the culture medium. At 17 h p.i., a time point at which GFP expression in untreated infected cells reaches its maximum for both viruses, cells were fixed, and GFP fluorescence was quantified.
The reporter gene expression of both SARS-CoV-GFP and EAV-GFP was already significantly inhibited in a dose-dependent manner by the addition of PT alone (Fig. 1B and C). This effect was significantly enhanced when 2 µM of Zn2+ was added to the medium. We found that addition of ZnOAc2 alone also reduced virus replication, but only at levels that were close to the 50% cytotoxicity concentration (CC50) of ZnOAc2 in Vero-E6 cells (∼70 µM, data not shown). This is likely due to the poor solubility of Zn2+ in phosphate-containing medium and the inefficient uptake of Zn2+ by cells in the absence of zinc-ionophores. The combination of 2 µM PT and 2 µM ZnOAc2 resulted in a 98±1% and 85±3% reduction of the GFP signal for EAV-GFP and SARS-CoV-GFP, respectively. No cytotoxicity was observed for this combination of PT and ZnOAc2 concentrations. From the dose-response curves in Fig. 1, a CC50 value of 82 µM was calculated for PT in the presence of 2 µM zinc. Half maximal inhibitory concentrations (IC50) of 1.4 µM and 0.5 µM and selectivity indices of 59 and 164 were calculated for SARS-CoV and EAV, respectively.
Zn2+ reversibly inhibits the RNA-synthesizing activity of isolated nidovirus RTCs
We previously developed assays to study the in vitro RNA-synthesizing activity of RTCs isolated from cells infected with SARS-CoV or EAV [25], [26]. In these RTC assays [α-32P]CMP is incorporated into both genomic (replication) and sg mRNA (transcription) (Fig. 2). This allowed us to monitor the synthesis of the same viral RNA molecules that can be detected by hybridization of RNA from nidovirus-infected cells. A benefit of these assays is that the activity does not depend on continued protein synthesis and that it allows us to study viral RNA synthesis independent of other aspects of the viral replicative cycle [26]. To investigate whether the inhibitory effect of PT and zinc ions on nidovirus replication in cell culture is reflected in a direct effect of Zn2+ on viral RNA synthesis, we tested the effect of Zn2+ addition on RTC activity. For both EAV (Fig. 2A) and SARS-CoV (Fig. 2B), a dose-dependent decrease in the amount of RNA synthesized was observed when ZnOAc2 was present. For both viruses, a more than 50% reduction of overall RNA-synthesis was observed at a Zn2+ concentration of 50 µM, while less than 5% activity remained at a Zn2+ concentration of 500 µM. Both genome synthesis and sg mRNA production were equally affected.
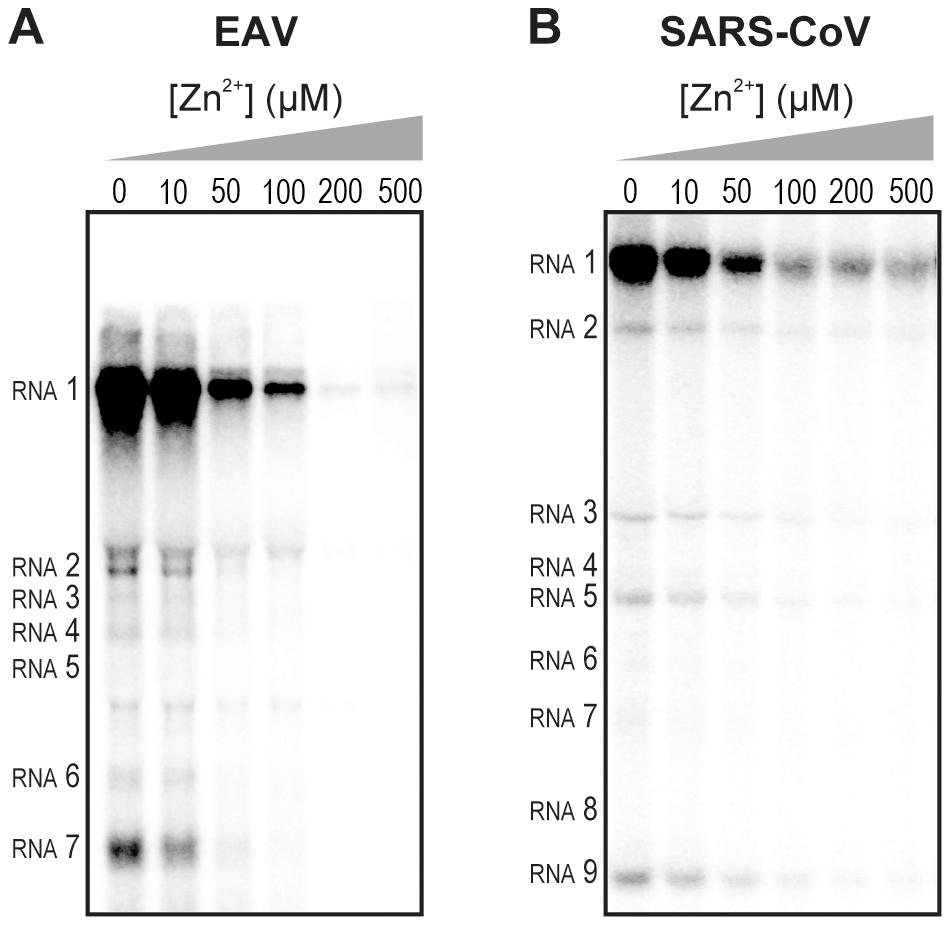
To test whether the inhibition of RTC activity by Zn2+ was reversible, RTC reactions were started in the presence or absence of 500 µM Zn2+. After 30 min, these reactions were split into two aliquots and magnesium-saturated EDTA (MgEDTA) was added to one of the tubes to a final concentration of 1 mM (Fig. 3A). We used MgEDTA as Zn2+ chelator in these in vitro assays, because it specifically chelates Zn2+ while releasing Mg2+, due to the higher stability constant of the ZnEDTA complex. Uncomplexed EDTA inhibited RTC activity in all reactions (data not shown), most likely by chelating the Mg2+ that is crucial for RdRp activity [27], [28], whereas MgEDTA had no effects on control reactions without Zn2+ (Fig. 3B, compare lane 1 and 2). As shown in Fig. 2, the EAV RTC activity that was inhibited by Zn2+ (Fig. 3B&C, lane 3) could be restored by the addition of MgEDTA (Fig. 3B, lane 4) to a level observed for control reactions without Zn2+ (Fig. 3B, lane 1). Compared to the untreated control, the EAV RTC assay produced approximately 30% less RNA, which was consistent with the 30% shorter reaction time after the addition of the MgEDTA (100 versus 70 min for lanes 1 and 4, respectively). Surprisingly, SARS-CoV RTC assays that were consecutively supplemented with Zn2+ and MgEDTA incorporated slightly more [α-32P]CMP compared to untreated control reactions (Fig. 3C; compare lane 1 and 4). This effect was not due to chelation of the Zn2+ already present in the post-nuclear supernatant (PNS) of SARS-CoV-infected cells, as this increase was not observed when MgEDTA was added to a control reaction without additional Zn2+ (Fig. 3C, lane 2).

Zinc ions affect the in vitro activity of recombinant nidovirus RdRps
To establish whether inhibition of RTC activity might be due to a direct effect of Zn2+ on nidovirus RdRp activity, we tested the effect of Zn2+ on the activity of the purified recombinant RdRps of SARS-CoV (nsp12) and EAV (nsp9) using previously developed RdRp assays [27], [28]. Using an 18-mer polyU template, the EAV RdRp incorporated [α-32P]AMP into RNA products of up to 18 nt in length (Fig. 4A). Initiation was de novo, which is in line with previous observations and the presence of a conserved priming loop in the nsp9 sequence [28]. Unlike the EAV RdRp nsp9, the in vitro activity of the SARS-CoV RdRp nsp12 – which lacks a priming loop – was shown to be strictly primer-dependent [27]. Thus, to study the RdRp activity of SARS-CoV nsp12, a primed polyU template was used (Fig. 4B), thereby allowing us to sample [α-32P]AMP incorporation as described previously [27]. As specificity controls, we used the previously described SARS-CoV nsp12 mutant D618A [27], which contains an aspartate to alanine substitution in motif A of the RdRp active site, and EAV nsp9-D445A, in which we engineered an aspartate to alanine substitution at the corresponding site of EAV nsp9 [28], [31]. Both mutant RdRps showed greatly reduced [α-32P]AMP incorporation in our assays (Fig. 4), confirming once again that the radiolabeled RNA products derived from nidovirus RdRp activity.

Addition of ZnOAc2 to RdRp assays resulted in a strong, dose-dependent inhibition of enzymatic activity for both the EAV and SARS-CoV enzyme (Fig. 5A and B, respectively), similar to what was observed in RTC assays. In fact, compared to other divalent metal ions such Co2+ and Ca2+, which typically bind to amino acid side chains containing oxygen atoms rather than sulfur groups, Zn2+ was the most efficient inhibitor of SARS-CoV nsp12 RdRp activity (Supplemental Fig. S1). To test whether, as in the RTC assay, the RdRp inhibition by zinc ions was reversible, RdRp assays were pre-incubated with 6 mM Zn2+, a concentration that consistently gave >95% inhibition. After 30 min, 8 mM MgEDTA was added to both a control reaction and the reaction inhibited with ZnOAc2, and samples were incubated for another 30 min (Fig. 5C). As shown in Fig. 5D, the inhibition of EAV RdRp activity by Zn2+ could be reversed by chelation of Zn2+ (Fig. 5D; compare lanes 3 and 4). The amount of product synthesized was consistently 60±5% of that synthesized in a 60-min control reaction (Fig. 5D; compare lanes 1 and 4), which was within the expected range given the shorter reaction time. The inhibition of the SARS-CoV RdRp was reversible as well. During the 30-min incubation after the addition of MgEDTA, SARS-CoV nsp12 incorporated 40±5% of the label incorporated during a standard 60-min reaction (Fig. 5E). This was slightly lower than the expected yield and may be caused by the elevated Mg2+ concentration, which was shown to be suboptimal for nsp12 activity [27] and results from the release of Mg2+ from MgEDTA upon chelation of Zn2+.

Differential effect of Zn2+ on the initiation and elongation phase of nidovirus RNA synthesis
For EAV, close inspection of the RdRp assays revealed a less pronounced effect of Zn2+ on the generation of full-length 18-nt products than on the synthesis of smaller reaction intermediates (Fig. 5A). This suggested that Zn2+ specifically inhibited the initiation step of EAV RNA synthesis. To test this hypothesis, an RTC assay was incubated for 30 min with unlabeled CTP (initiation), after which the reaction was split in two. Then, [α-32P]CTP was added to both tubes (pulse), 500 µM Zn2+ was added to one of the tubes, and samples were taken at different time points during the reaction (Fig. 6A). Fig. 6B shows that in the presence of Zn2+ [α-32P]CMP was predominantly incorporated into nascent RNA molecules that were already past the initiation phase at the moment that Zn2+ was added to the reaction. No new initiation occurred, as was indicated by the smear of short radiolabeled products that progressively shifted up towards the position of full-length genomic RNA. This suggested that Zn2+ does not affect the elongation phase of EAV RNA synthesis and that it specifically inhibits initiation. This also explains the relatively weak signal intensity of the smaller sg mRNA bands (e.g., compare the relative change in signal of RNA2 to RNA7) produced in the presence of Zn2+, since multiple initiation events are required on these short molecules to obtain signal intensities similar to those resulting from a single initiation event on the long genomic RNA, e.g., 16 times more in the case of RNA7. In contrast to EAV, the effect of Zn2+ on RNA synthesis by SARS-CoV RTCs was not limited to initiation, but appeared to impair the elongation phase as well, given that the addition of Zn2+ completely blocked further incorporation of [α-32P]CMP when added 40 min after the start of the reaction (Fig. 6C).

In the RdRp assays, the short templates used made it technically impossible to do experiments similar to those performed with complete RTCs. However, we previously noticed that at low concentrations of [α-32P]ATP (∼0.17 µM) SARS-CoV nsp12 RdRp activity was restricted to the addition of only a single nucleotide to the primer [27]. EAV nsp9 mainly produced very short (2–3 nt long) abortive RNA products and only a fraction of full length products, as is common for de novo initiating RdRps [28]. This allowed us to separately study the effect of Zn2+ on initiation and elongation by performing an experiment in which a pulse with a low concentration of [α-32P]ATP was followed by a chase in the presence of 50 µM of unlabeled ATP, which increased processivity and allowed us to study elongation (Fig. 7A and C) as described previously [27]. The results of these experiments were in agreement with those obtained with isolated RTCs. For EAV, with initiation and dinucleotide synthesis completely inhibited by the presence of 6 mM Zn2+ (Supplemental Fig. S2A), the amount of reaction intermediates shorter than 18 nt diminished with time, while products from templates on which the RdRp had already initiated were elongated to full-length 18-nt molecules (Fig. 7B, right panel). This was consistent with the observation that the EAV RdRp remained capable of extending the synthetic dinucleotide ApA to trinucleotides in the presence of Zn2+ (Supplemental Fig. S2B). Likely due to the absence of reinitiation in the reactions shown in Fig. 7B, the low processivity of the EAV RdRp, and the substrate competition between the remaining [α-32P]ATP and the >200 fold excess of unlabeled ATP, the differences between the 5- and 30-min time points were small. In the absence of Zn2+, the RdRp continued to initiate as indicated by the ladder of smaller-sized RNA molecules below the full-length product (Fig. 7B, left panel) and the time course shown in Supplemental Fig. S2A. In contrast, the addition of Zn2+ to a SARS-CoV RdRp reaction also blocked elongation, since extension of the radiolabeled primer as observed in the absence of Zn2+ (Fig. 7D, left panel) no longer occurred (Fig. 7D, right panel).

Zinc affects SARS-CoV RdRp template binding
To assess whether Zn2+ affects the interaction of recombinant SARS-CoV nsp12 with the template used in our assays, we performed electromobility shift assays (EMSA) in the presence and absence of Zn2+ (Fig. 8A). To measure the binding affinity of the RdRp for the template, we determined the fraction of bound template at various protein concentrations and observed a 3–4 fold reduction in RNA binding when Zn2+ was present in the assay (Fig. 8B). We also assessed whether pre-incubation of the RdRp or RNA with Zn2+ was a requirement for this drop in binding affinity, but found no significant difference with experiments not involving such a preincubation (data not shown).

No binding was observed when a similar RNA binding assay was performed with purified EAV RdRp. Likewise, nsp9 did not bind RNA in pull-down experiments with Talon-beads, His6-tagged nsp9, and radiolabeled EAV genomic RNA or various short RNA templates including polyU, whereas we were able to detect binding of a control protein (SARS-CoV nsp8, which has demonstrated RNA and DNA binding activity [32]) using this assay. It presently remains unclear why we are not able to detect the binding of recombinant EAV nsp9 to an RNA template.
Discussion
Although a variety of compounds have been studied, registered antivirals are currently still lacking for the effective treatment of SARS and other nidovirus-related diseases [33]. RdRps are suitable targets for antiviral drug development as their activity is strictly virus-specific and may be blocked without severely affecting key cellular functions. Several inhibitors developed against the polymerases of e.g. human immunodeficiency virus (HIV) and hepatitis C virus are currently being used in antiviral therapy or clinical trials [34], [35], [36]. Therefore, advancing our molecular knowledge of nidovirus RdRps and the larger enzyme complexes that they are part of, and utilizing the potential of recently developed in vitro RdRp assays [25], [26], [27], [28] could ultimately aid in the development of effective antiviral strategies.
Zinc ions and zinc-ionophores, such as PT and PDTC, have previously been described as potent inhibitors of various RNA viruses. We therefore investigated whether PT-stimulated import of zinc ions into cells also inhibited the replication of nidoviruses in cell culture. Using GFP-expressing EAV and SARS-CoV [29], [30], we found that the combination of 2 µM PT and 2 µM Zn2+ efficiently inhibited their replication, while not causing detectable cytoxicity (Fig. 1). Inhibition of replication by PT and Zn2+ at similar concentrations (2–10 µM) was previously observed for several picornaviruses such as rhinoviruses, foot-and-mouth disease virus, coxsackievirus, and mengovirus [6], [7], [8], [9], [10], [11].
The inhibitory effect of Zn2+ on the replication of picornaviruses appeared to be due to interference with viral polyprotein processing. In infections with the coronavirus mouse hepatitis virus (MHV), Zn2+ also interfered with some of the replicase polyproteins cleavages [24], albeit at a much higher concentration (100 µM Zn2+) than used in our studies. Since impaired replicase processing will indirectly affect viral RNA synthesis in the infected cell, we used two recently developed in vitro assays to investigate whether Zn2+ also affects nidovirus RNA synthesis directly. Our in vitro studies revealed a strong inhibitory effect of zinc ions on the RNA-synthesizing activity of isolated EAV and SARS-CoV RTCs. Assays with recombinant enzymes subsequently demonstrated that this was likely due to direct inhibition of RdRp function. The inhibitory effect could be reversed by chelating the zinc ions, which provides an interesting experimental (on/off) approach to study nidovirus RNA synthesis. Addition of Zn2+ following initiation of EAV RNA synthesis had little or no effect on NTP incorporation in molecules whose synthesis had already been initiated in the absence of Zn2+ (Fig. 6 and 7), indicating that Zn2+ does not affect elongation and does not increase the termination frequency, as was previously found for Mn2+ [25]. Therefore, Zn2+ appears to be a specific inhibitor of the initiation phase of EAV RNA synthesis. In contrast, Zn2+ inhibited SARS-CoV RdRp activity also during the elongation phase of RNA synthesis, probably by directly affecting template binding (Fig. 8). In coronaviruses, zinc ions thus appear to inhibit both the proper proteolytic processing of replicase polyproteins [23], [24] and RdRp activity (this study). Contrary to the RTC assays, millimolar instead of micromolar concentrations of ZnOAc2 were required for a nearly complete inhibition of nucleotide incorporation in RdRp assays.
It has been well established that DNA and RNA polymerases use a triad of conserved aspartate residues in motifs A and C to bind divalent metal ions like Mg2+, which subsequently coordinate incoming nucleotides during the polymerization reaction [37], [38]. Mg2+ is also the divalent metal ion that is required for the in vitro activity of isolated SARS-CoV and EAV RTCs and recombinant RdRps [25], [26], [27], [28], although de novo initiation of EAV nsp9 is primarily Mn2+-dependent. Zn2+ could not substitute for Mg2+ or Mn2+ as cofactor as it was incapable of supporting the polymerase activity of nidovirus RTCs and RdRps in the absence of Mg2+ (data not shown), as was also reported for the poliovirus RdRp [39]. Moreover, inhibition of nidovirus RdRp activity by Zn2+ was even observed at low concentrations and in the presence of a more than 25-fold excess of Mg2+, suggesting that either the affinity of the active site for Zn2+ is much higher or that Zn2+ does not compete for Mg2+-binding and binds to another zinc(-specific) binding site in the protein.
Specific protein domains or pockets that contain zinc ions may be involved in protein-protein interactions, protein-RNA/DNA interactions, or conformational changes in enzyme structures. Zinc-binding domains commonly consist of at least three conserved cysteine and/or histidine residues within a stretch of ∼10–30 amino acids, such as in zinc-finger motifs and metalloproteases [2], [40], [41]. However, in RdRps there are only few precedents for the presence of zinc-binding pockets, such as those identified in the crystal structure of the Dengue RdRp [42]. Sequence analysis of the EAV nsp9 amino acid sequence revealed that it lacks patches rich in conserved cysteines and/or histidines. In contrast, inspection of the SARS-CoV nsp12 amino acid sequence revealed two such patches, namely H295-C301-C306-H309-C310 and C799-H810-C813-H816. A crystal structure for nsp12 is presently unavailable, but a predicted structure that represents the C-terminal two-thirds of the enzyme has been published [31]. Interestingly, in this model, C799, H810, C813 and H816 are in a spatial arrangement resembling that of the Zn2+ coordinating residues in the Zn2 zinc-binding pocket found in motif E of the Dengue virus RdRp (see Supplemental Fig. S3). Clearly, an in-depth analysis of nidovirus RdRps, e.g. through structural analysis and subsequent mutational studies targeting aforementioned cysteines and histidines, is required to provide further insight into and a structural basis for the Zn2+-induced inhibitory effects on RdRp activity documented in this study. Such studies may, however, be complicated when Zn2+ binding proves to be very transient in nature and not detectable with currently available methods.
In summary, the combination of zinc ions and the zinc-ionophore PT efficiently inhibits nidovirus replication in cell culture. This provides an interesting basis for further studies into the use of zinc-ionophores as antiviral compounds, although systemic effects have to be considered [43], [44] and a water-soluble zinc-ionophore may be better suited, given the apparent lack of systemic toxicity of such a compound at concentrations that were effective against tumors in a mouse xenograft model [45]. In vitro, the reversible inhibition of the RdRp by Zn2+ has also provided us with a convenient research tool to gain more insight into the molecular details of (nido)viral RNA synthesis, and revealed novel mechanistic differences between the RdRps of SARS-CoV and EAV.
MATERIALS AND METHODS
Cells and viruses
Vero-E6 cells were cultured and infected with SARS-CoV (strain Frankfurt-1; accession nr. AY291315) or SARS-CoV-GFP as described previously [46]. All procedures involving live SARS-CoV were performed in the biosafety level 3 facility at Leiden University Medical Center. BHK-21 or Vero-E6 cells were cultured and infected with EAV (Bucyrus strain; accession nr. NC_002532) or EAV-GFP [29] as described elsewhere [25].
Effect of zinc ions on nidovirus replication in cell culture
One day prior to infection, Vero-E6 cells were seeded in transparent or black (low fluorescence) 96-well clusters at 10,000 cells per well. The next day, cells were infected with SARS-CoV-GFP or EAV-GFP with an m.o.i. of 4, and 1 h p.i. the inoculum was removed and 100 µl of medium containing 2% fetal calf serum (FCS) was added to each well. In some experiments 0–32 µM of pyrithione (Sigma) was added in addition to 0–2 µM ZnOAc2. Infected cells were fixed at 17 h p.i. by aspirating the medium and adding 3% paraformaldehyde in PBS. After washing with PBS, GFP expression was quantified by measuring fluorescence with a LB940 Mithras plate reader (Berthold) at 485 nm. To determine toxicity of ZnOAc2 and PT, cells were exposed to 0–32 µM PT and 0–8 µM ZnOAc2. After 18 h incubation, cell viability was determined with the Cell Titer 96 AQ MTS assay (Promega). EC50 and CC50 values were calculated with Graphpad Prism 5 using the nonlinear regression model.
RNA templates and oligonucleotides
RNA oligonucleotides SAV557R (5′-GCUAUGUGAGAUUAAGUUAU-3′), SAV481R (5′-UUUUUUUUUUAUAACUUAAUCUCACAUAGC-3′) and poly(U)18 (5′-UUUUUUUUUUUUUUUUUU-3′) were purchased from Eurogentec, purified from 7 M Urea/15% PAGE gels and desalted through NAP-10 columns (GE healthcare). To anneal the RNA duplex SAV557R/SAV481R, oligonucleotides were mixed at equimolar ratios in annealing buffer (20 mM Tris-HCl pH 8.0, 50 mM NaCl and 5 mM EDTA), denatured by heating to 90°C and allowed to slowly cool to room temperature after which they were purified from 15% non-denaturing PAGE gels.
In vitro viral RNA synthesis assay with isolated RTCs
SARS-CoV and EAV RTCs were isolated from infected cells and assayed for activity in vitro as described previously [25], [26]. To assess the effect of Zn2+, 1 µl of a ZnOAc2 stock solution was added to standard 28-µl reactions, resulting in final Zn2+ concentrations of 10–500 µM. When Zn2+ had to be chelated in the course of the reaction, magnesium-saturated EDTA (MgEDTA) was added to a final concentration of 1 mM. After RNA isolation, the 32P-labeled reaction products were separated on denaturing 1% (SARS-CoV) or 1.5% (EAV) agarose formaldehyde gels. The incorporation of [α-32P]CMP into viral RNA was quantified by phosphorimaging of the dried gels using a Typhoon scanner (GE Healthcare) and the ImageQuant TL 7 software (GE Healthcare).
Expression and purification of nidovirus RdRps
SARS-CoV nsp12 and EAV nsp9 were purified essentially as described elsewhere [27], [28], but with modifications for nsp9. In short, E. coli BL21(DE3) with plasmid pDEST14-nsp9-CH was grown in auto-induction medium ZYM-5052 [47] for 6 hours at 37°C and a further 16 hours at 20°C. After lysis in buffer A (20 mM HEPES pH 7.4, 200 mM NaCl, 20 mM imidazole, and 0.05% Tween-20) the supernatant was applied to a HisTrap column (GE Healthcare). Elution was performed with a gradient of 20–250 mM imidazole in buffer A. The nsp9-containing fraction was further purified by gel filtration in 20 mM HEPES, 300 mM NaCl and 0.1% Tween-20 on a Superdex 200 column (GE Healthcare). The fractions containing nsp9-CH were pooled, dialyzed against 1000 volumes of buffer B (20 mM HEPES, 100 mM NaCl, 1 mM DTT and 50% glycerol) and stored at −20°C. RdRps with a D618A (SARS-CoV) or D445A (EAV) mutation were obtained by site-directed mutagenesis of the wild-type (wt) plasmid pDEST14-nsp9-CH [28] with oligonucleotides 5′-TACTGCCTTGAAACAGCCCTGGAGAGTTGTGAT-3′ and 5′-ATCACAACTCTCCAGGGCTGTTTCAAGGCAGTA-3′, and plasmid pASK3-Ub-nsp12-CHis6 with oligonucleotides 5′-CCTTATGGGTTGGGCTTATCCAAAATGTG-3′ and 5′-CACATTTTGGATAAGCCCAACCCATAAGGA-3′, as described elsewhere [27]. Mutant proteins were purified parallel to the wt enzymes.
RdRp assays with purified enzymes
Standard reaction conditions for the RdRp assay with 0.1 µM of purified SARS-CoV nsp12 are described elsewhere [27]. To study the effect of Zn2+ in this assay, 0.5 µl of a dilution series of 0–80 mM ZnOAc2 was added to the 5 µl reaction mixture, yielding final Zn2+ concentrations of 0–8 mM. The EAV RdRp assay contained 1 µM nsp9, 1 µM RNA template poly(U)18, 0.17 µM [α-32P]ATP (0.5 µCi/µl; Perkin-Elmer), 50 µM ATP, 20 mM Tris-HCl (pH 8.0), 10 mM NaCl, 10 mM KCl, 1 mM MnCl2, 4 mM MgOAc2, 5% glycerol, 0.1% Triton-X100, 1 mM DTT and 0.5 units RNaseOUT. ZnOAc2 was added to the reaction to give a final concentration of 0–6 mM. To chelate Zn2+ during reactions, MgEDTA was added to a final concentration of 8 mM. Reactions were terminated after 1 hour and analyzed as described [27].
SARS-CoV nsp12 electrophoretic mobility shift assay
SARS-CoV RdRp was incubated with 0.2 nM 5′ 32P-labeled SAV557R/SAV481R RNA duplex, for 10 minutes at 30°C either in presence or absence of 6 mM ZnOAc2. Reactions were analyzed as described previously [27].
If you go to the store to buy Meat, don't run to the Milk section or the Junk Food aisle looking for it!!
The Meat Section is the True Gospel of Jesus Christ.
The Milk Section is likened to those who will not preach on sin and Hell, just a feel good message, the Social gospel.
The Junk Food Isle is the outright false doctrine AKA the prosperity gospel, name it and claim it, the Hebraic Roots movement and other false teachings!!
Feasting on just Milk and Junk will eventually cause you great harm, you can count on it!!
If you appreciate what this Ministry is doing to Expose the Fake Christians, Satanists, Witches, Communist/Socialist Democrats, R.I.N.O Republicans and the assault on our Conservative, True Christian values, please consider a small donation to help us continue and expand. This Ministry is not only under attack by the Enemy, we are now under attack from supposed Christians also. It is what Tom Horn calls 'Blood on the Altar"!